So the fluid level has been slowly dropping in your supposedly maintenance free battery, but you've never noticed a loss of power. That's because it hasn't dropped significantly below the level of the tops of the plates, where the power is made. But here is an often-forgotten fact about battery construction: the lead bars that connect to the plates and run between the cells are just above that level. If the fluid level should fall below these bars and expose them to air, the darned things start corroding for some reason, especially if the car sits for a while and the fluid doesn't slosh up on the lead and keep it wet. After corroding a while, the darned bars don't work, and sometimes even make a spark which blows up the battery!
The lesson here is that maintenance free batteries... aren't. If you're like me and you lose the battery receipts after a couple of years, then find out how to open those batteries up and make sure you top them off with distilled water. Usually you can peel off the label or something like that and there are some caps under there.
If you've ever charged a battery until it gassed, you should check the fluid level in it. Never add anything but distilled water! Adding acid is not going to "freshen" the battery; it will usually ruin it. Adding dirty water is going to make minerals settle out and accumulate in the bottom of the battery, which often shorts out the plates, or other untoward things could happen with impure water. I use only reverse osmosis water; don't use "baby mineral water" or the like from the store. Sufficiently distilled water is fine.
If the battery will hardly take a charge at all, then it's "sulfated"; there is an insulating layer of sulfate on the plates. This happens to any battery that sits around in a discharged condition. A battery that is heavily sulfated is going to be difficult to revive by just charging it. There are electronic and chemical ways to get the sulfate off, though.
Sending pulses of high voltage/current through a battery will slowly knock sulfates off of the plates. I have had pretty decent luck with batteries as long as they're mechanically sound (the plates aren't broken, shorted, etc.). The schematic for a desulfator may be found here. If you build one, remember the error in the original article about the capacitor having the wrong value printed: the .022uf should be .0022uf instead. If the plates look black, crumbly, and ugly, then the desulfating isn't going to work very well, but if there's an obvious layer of white sulfate on the plates, you'll probably have good luck.
Tetrasodium EDTA (about $15 per pound, plus shipping, from www.bostick-sullivan.com) is a chemical way to remove sulfates. Some people just dump it into the battery (it's in powder form) but that gives the sulfates a chance to precipitate out of solution and settle in the bottom of the battery. I'd prefer to get those nasty sulfates out of the battery totally.
I mix a solution of 1 tablespoon to one quart of water. Empty the acid from the battery; I usually turn the battery upside down in a sufficiently large pail or 5-gallon bucket. Try to keep the acid clean, as you'll probably want to reuse it. Rinsing the battery out with distilled water can't hurt either. Please note that fluid/acid/water will "stick" between the battery plates, especially when that space is filled up with sulfates, and it can be difficult to get all of it out! A bit of shaking is sometimes warranted, or maybe several rinses with distilled water.
I refill the battery with the EDTA solution and leave it overnight. This is a fairly weak solution, and you may wish to double the strength for this purpose. Also, keep the solution away from light so it won't break down.
The next day the solution should look milky when you pour it out of the battery. You've made progress! I don't think this "used" solution is good for anything, but if I think of something I'll post it here. Anyway, repeat the process until the battery is noticably cleaner (the white sulfate on the plates should be disappearing). Sometimes one cell will be much more sulfated than others, and you may wish to treat that cell only. Heat will speed up the process, although I wouldn't get too carried away trying to heat up your batteries!
When the process is done, put the old acid back in, unless you happen to have new acid kicking around. If you do have new acid, then make sure it's properly mixed with water. Buy a hygrometer (Wal-Mart has cool ones for $1 lately) so you know for sure what's going on.
Another trick I've heard of for reviving batteries is to drain/save the acid, fill them with distilled water, and charge them (overnight, at low current). Do this 2-3 times and it might take some sulfates off of the plates. I'll check into this as it might not be very effective.
I think the most reasonable approach for heavily sulfated batteries will be to treat them chemically, then leave them pulsing with the electronic device for a while.
I've heard that lots of batteries come with EDTA in them now. Doubtless there are other chemical tricks/processes which can make a battery perform well. But the EDTA trick is easy enough for the common man to implement. Adding a small amount of EDTA to each cell is supposed to do the battery a world of good over the long run. It doesn't dissolve well in battery acid, so mixing in a little water couldn't hurt -- but even just dropping the powder in works as a preventative measure, and I'm sure it dissolves just fine over time. Search the internet for suggested dosages; it doesn't take much.
The electronic desulfator presents a very small load to the battery and really doesn't have any undesirable effects that I know of. Probably the more that you can run these devices, the better, at least on batteries that are used only occasionally and more likely to develop sulfates.
You need to keep your batteries charged... but you don't want to overcharge them. There are automatic chargers which do a pretty good job of detecting battery charge and keeping them at the right level. Keep in mind that small "tickle chargers" are not in any way "automatic" and you can certainly overcharge a battery with one of these if you leave it on long enough.
Not all lead-acid batteries have exactly the same voltage curve, but 12V batteries should be close enough so that they work well paralleled. There will be some resistance due to wire lengths/sizes in a system such as this, of course, but you can certainly expect the batteries to stay reasonably charged over the long wire, and if you want to pull a heavy load from one end or another, just make sure that you have batteries on that end for that purpose.
It's useful to have a good digital meter to check your battery voltages with, but you can't usually leave your digital meter hooked up and turned on all the time. Analog meters can be a bit inaccurate, though, since the range of voltages you want to read (10-15 volts) will be 1/3 or less of the meter's scale. We can improve upon the accuracy and usefulness of such a meter by dispensing with the lower range of voltages (up to 9-10 volts) and displaying only the range we're interested in.
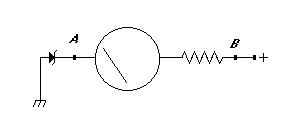
Here is a schematic for a meter which starts reading at 9 volts. You can get a 9V zener diode at Radio Shack for a dollar or so. The resistor on the right will depend on your meter movement. Many movements have the full-scale current written on them somewhere, and you can figure out an appropriate value based upon Ohm's law. Try something around 100k to start with if you're clueless about your meter's sensitivity.
This circuit will also work with a regular voltmeter (of any sensitivity) if you simply replace the meter movement and resistor in the below circuit with the meter in question.
If your meter is too sensitive (it's possible) then the zener diode will not reach rated voltage. If the circuit doesn't read correctly, then add a 10k or so resistor between points A and B. Calibrate the meter by comparing readings with a digital meter and choosing an appropriate resistor for the full-scale voltage you want (around 15V seems fine). After you have an appropriate resistor, make marks on your meter with a felt-tip or piece of tape or something.
This circuit will draw a small current from your batteries, and so we're assuming that you want to install it on a working system, one which maintains the charge of the batteries somehow, and one on which you need to check the voltage on a regular basis.

This is a pretty darned basic schematic, and I'll try to add some more schematics here soon. I guess I oughta find one of those schematic-drawing programs on the internet!